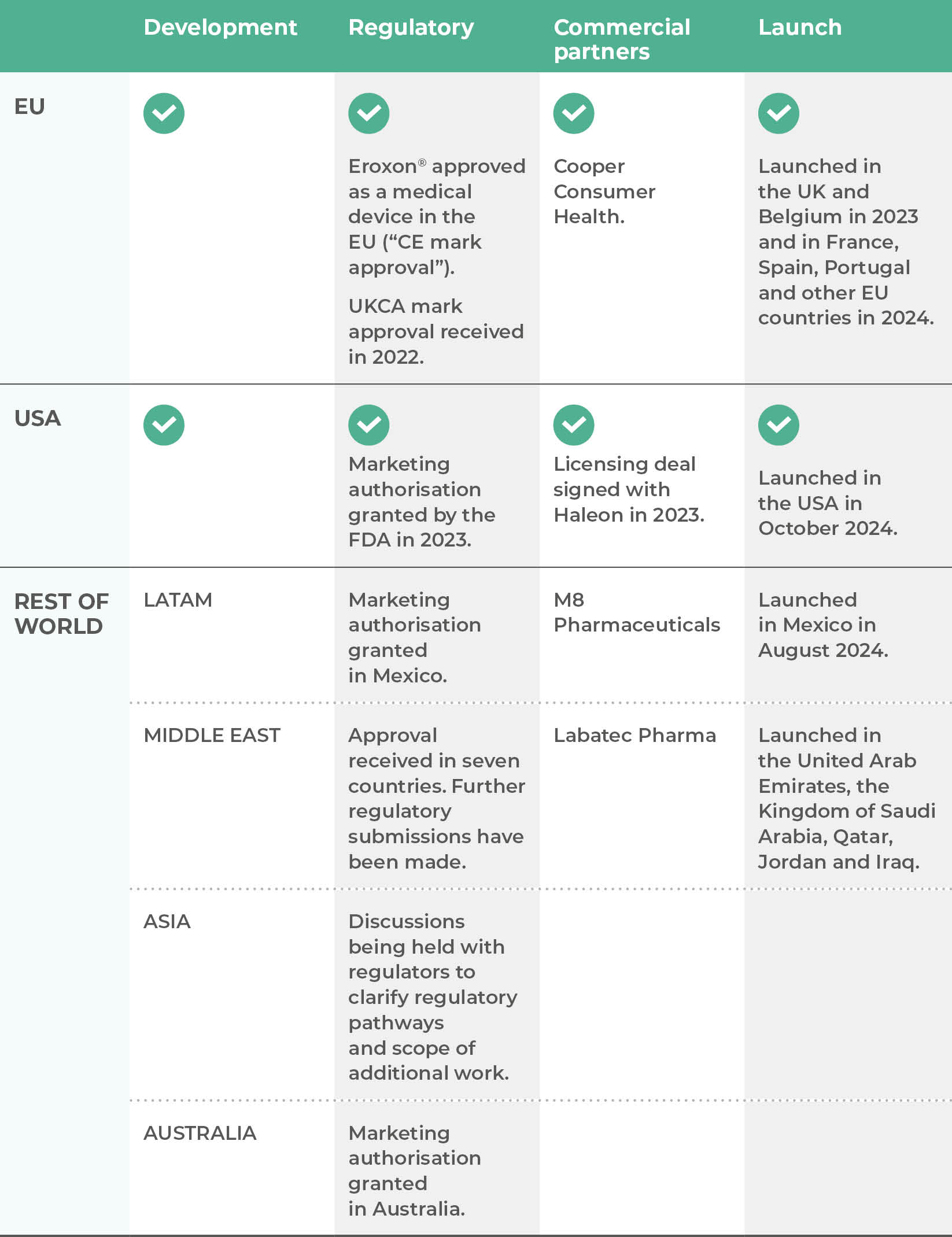
Futura’s lead product is Eroxon®, a unique topical treatment for erectile dysfunction which has been approved without the need for a prescription in many countries around the world, including in the USA and Europe and which has launched in a number of countries in Europe including the UK.
Eroxon® at a glance